Background: Despite the efficacy of covalent (c) Bruton tyrosine kinase inhibitors (BTKi) in R/R MCL, disease relapse arises through evolution of resistance mechanisms or development of cBTKi intolerance. Pirtobrutinib, a highly selective, non-covalent (reversible) BTKi has favorable oral pharmacology that enables continuous BTK inhibition throughout the daily dosing interval regardless of the intrinsic rate of BTK turnover. Pirtobrutinib is the first BTKi to demonstrate durable efficacy following prior cBTKi therapy in heavily pre-treated R/R MCL and was well-tolerated with a low frequency of treatment discontinuation due to toxicity (Wang et al., JCO, 2023). Pirtobrutinib is approved in the USA to treat relapsed or refractory MCL after at least two lines of systemic therapy including a prior cBTKi. Here, we report updated results of pirtobrutinib therapy in all patients (pts), including those with biologically high-risk R/R MCL with a median survival follow-up of 24.2 months (range, 18.2-29.8).
Methods: Pts with R/R MCL received pirtobrutinib monotherapy in the multicenter Phase 1/2 BRUIN trial (NCT03740529). Efficacy was assessed in all cBTKi pre-treated pts, as well as in cBTKi treatment-naïve pts. Key endpoints included overall response rate (ORR) as assessed by independent review committee per Lugano 2014 criteria, duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety. Pts were included across the dose escalation range and expansion (25-300 mg/day) with 93% (n=141) receiving at least one dose of 200 mg/day, the FDA-approved dose. A data cut of 05 May 2023 was utilized.
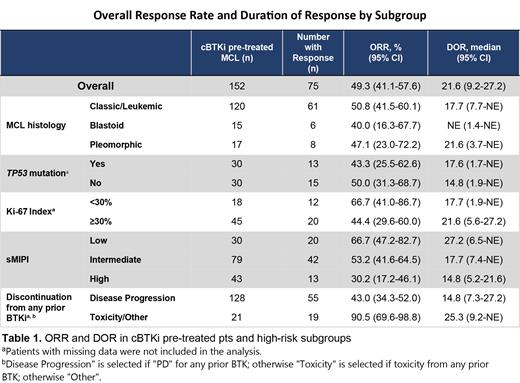
Results: Among all 152 pts with R/R MCL who received a prior cBTKi, the median age was 70 years (range, 46-88), and 52% had intermediate-risk and 28.3% had high-risk sMIPI scores. Median prior lines of therapy were 3 (range, 1-9), including an anti-CD20 antibody (96.7%), chemotherapy (90.1%), immunomodulator (17.1%), stem cell transplant (21.7%), BCL-2 inhibitor (15.8%), CAR-T cell therapy (8.6%), and PI3K inhibitor (3.9%). Among pts with high-risk biomarker data available, 30/60 (50%) had TP53 mutations and 45/63 (71%) had a Ki-67 index of ≥30%. The ORR for cBTKi pre-treated pts was 49.3% (95% CI, 41.1-57.6), including 15.8% complete responses (n=24) and 33.6% partial responses (n=51), whilst cBTKi naïve pts (n=14) had an ORR of 85.7% (95% CI, 57.2-98.2). The ORR among 128 pts who had discontinued a prior cBTKi due to PD and 21 pts who had discontinued for toxicity/other reasons was 43.0% and 90.5%, respectively. Among the 75 responding cBTKi pre-treated pts, the median DOR was 21.6 months (95% CI, 9.2-27.2) at a median follow-up of 24 months. The 18- and 24-month DOR rates were 51.9% (95% CI, 37-64.8) and 38.9% (95% CI, 22.7-54.8), respectively. ORR and DOR by high-risk subgroups (including blastoid/pleomorphic variants, Ki-67 index ≥30%, and TP53 mutations) are shown in Table 1. The 18- and 24-month DOR rates among 12 responding cBTKi naïve pts were both 90.0% (95% CI, 47.3-98.5). The median PFS and OS for cBTKi pre-treated pts was 5.6 months (95% CI, 5.3-9.2), and 23.5 months (95% CI, 17.1-NE), respectively. In the MCL cohort (n=166), the most frequent treatment-emergent adverse events (TEAEs) were fatigue (31.9%), diarrhea (22.3%), and dyspnea (17.5%). The most common Grade ≥3 TEAE was neutropenia/neutrophil count decreased (13.3%) and the rate of Grade ≥3 infections was (19.9%). Grade ≥3 hemorrhage/hematoma (2.4%) and all-grade atrial fibrillation/flutter (3.6%) were infrequent. Overall, 8 pts (5%) had treatment-related AEs leading to dose reductions and 5 (3%) had treatment-related AEs leading to pirtobrutinib discontinuation.
Conclusion: Pirtobrutinib continues to demonstrate durable efficacy and a favorable safety profile in heavily pre-treated R/R MCL pts with prior cBTKi therapy. High ORRs were observed in pts who had PD on a prior cBTKi, and in pts with high-risk disease features including blastoid/pleomorphic variants, elevated Ki-67 index, and TP53 mutations.
OffLabel Disclosure:
Cohen:BMS/Celgene: Research Funding; Novartis: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Shah:Epizyme: Consultancy; LOXO-Lilly: Consultancy, Other: Travel support; Tundra Therapeutics: Current holder of stock options in a privately-held company; BMS/Juno: Consultancy; Novartis: Consultancy; Umoja: Consultancy; TG therapeutic: Consultancy; Janssen: Consultancy; Seattle Genetics: Consultancy; Gilead/Kite: Consultancy; Incyte: Consultancy; Abbvie: Consultancy; Lilly Oncology: Consultancy, Research Funding; Miltenyi Biotec: Consultancy, Other: Travel support, Research Funding. Jurczak:BeiGene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; Eli Lilly: Consultancy; Pfizer: Consultancy; Roche: Consultancy; SOBI: Consultancy; Takeda: Consultancy; AbbVie: Research Funding; AstraZeneca: Research Funding; Bayer: Research Funding; BeiGene: Research Funding; Celgene: Research Funding; Janssen: Research Funding; Eli Lilly: Research Funding; Merck: Research Funding; Pfizer: Research Funding; Roche: Research Funding; SOBI: Research Funding; Takeda: Research Funding. Zinzani:ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Cheah:Menarini: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; Ascentage Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZenecca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Dizal: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Eyre:Medscape: Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eli Lilly and Company: Consultancy, Honoraria, Speakers Bureau; KITE: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Autolus: Consultancy; Janssen: Consultancy, Honoraria, Speakers Bureau; PeerView: Speakers Bureau; Loxo Lilly: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Loxo Oncology: Consultancy, Honoraria, Other, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees. Ujjani:Beigene: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Atara: Consultancy; Abbvie: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Lilly: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy; Astrazeneca: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Other: Travel expenses , Research Funding; PCYC: Research Funding. Koh:BMS Korea: Consultancy; Takeda Korea: Consultancy; Janssen Korea: Consultancy; Novartis Korea: Consultancy; Deep Metrics: Current equity holder in private company; Curocell: Current equity holder in private company; Proteina: Current holder of stock options in a privately-held company; Tomocube: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Research Funding; Genome Opinion: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Kim:Sanofi: Research Funding; Kyowa-Kirin: Research Funding; Roche: Research Funding; Boryung: Research Funding; Donga: Research Funding; Beigene: Research Funding. Flinn:Myeloid Therapeutics: Consultancy; Century Therapeutics: Consultancy; Genmab: Consultancy; Novartis: Consultancy; Hutchinson MediPharma: Consultancy; BeiGene: Consultancy; AbbVie: Consultancy; Innocare Pharma: Consultancy; Kite: Consultancy; Servier Pharma: Consultancy; Genentech: Consultancy; Secura Bio: Consultancy; TG Therapeutics: Consultancy; Vincerx Pharma: Consultancy. Tessoulin:Incyte: Honoraria; Kite: Honoraria; Abbvie: Honoraria; Gilead: Honoraria. Ma:BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Research Funding; Juno/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Consultancy; Eli Lilly and Company/Loxo Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Alencar:Beigene: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Lilly: Membership on an entity's Board of Directors or advisory committees; Dr Reddy: Honoraria; Genentech: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; SeaGen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Lewis:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy, Membership on an entity's Board of Directors or advisory committees; Lilly: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Woyach:Newave: Consultancy; Loxo: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; Abbvie: Consultancy; Schrodinger: Research Funding; Morphosys: Research Funding; Karyopharm: Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding. Maddocks:Pharmacyclics: Consultancy, Research Funding; Gilead/Kite: Consultancy; BeiGene: Consultancy; Epizyme: Consultancy; Eli Lilly and Company: Consultancy; Seattle Genetics: Consultancy; Novartis: Research Funding; Merck: Research Funding; AbbVie: Consultancy; AstraZeneca: Consultancy, Research Funding; Morphosys: Consultancy; Janssen: Consultancy, Honoraria; GenMab: Consultancy; Genentech: Consultancy; Incyte: Consultancy, Honoraria; ADC Therapeutics: Consultancy; Celgene: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Patel:Loxo Oncology: Consultancy, Research Funding; Fate Therapeutics: Research Funding; Genentech/Roche: Consultancy, Research Funding; Kite: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; ADC Therapeutics: Consultancy; Pharmacyclics/Janssen: Consultancy, Research Funding; Nurix: Research Funding; Morphosys: Consultancy; Merck: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; Epizyme: Consultancy, Research Funding; Curis, Inc: Research Funding; CRISPR Therapeutics: Research Funding; Caribou Biosciences: Consultancy; BeiGene: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Xencor: Consultancy, Research Funding; Trillium Therapeutics/Pfizer: Consultancy, Research Funding; TG Therapeutics: Consultancy, Speakers Bureau; Sunesis Pharmaceuticals: Research Funding; Adaptive Biotechnologies: Research Funding; Abbvie: Consultancy. Wang:Genentech: Research Funding; Morphosys: Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Rhodes:Velosbio: Research Funding; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy; Genentech: Consultancy; AbbVie: Consultancy, Research Funding; AstraZeneca: Consultancy; Morphosys: Consultancy; ADCT: Consultancy; Acerta: Research Funding; Epizyme: Consultancy; Loxo Oncology: Research Funding; Oncternal Pharmaceuticals: Research Funding; Verastem: Consultancy; PCYC: Consultancy; SeaGen: Consultancy; GenMab: Consultancy; Beigene: Consultancy. Tam:Novartis: Honoraria; Roche: Honoraria; LOXO: Honoraria; BeiGene: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Seymour:BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Hoffmann-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Research Funding; Genor Bio: Membership on an entity's Board of Directors or advisory committees. Nagai:Novartis: Honoraria; Mundi pharma: Honoraria; MSD: Honoraria, Research Funding; Solasia: Research Funding; Daiichi Sankyo: Research Funding; Mitsubishi Tanabe: Research Funding; Takeda: Honoraria, Research Funding; Eli Lilly: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Eisai: Honoraria; Meiji Seika Pharma: Honoraria; Sumitomo Pharma: Honoraria; GSK: Honoraria; Ono: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Celgene: Research Funding; Zenyaku Kogyo: Research Funding; BMS: Honoraria; Astra Zeneka: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; HUYA: Research Funding; Genmab: Honoraria, Research Funding; Beigene: Research Funding. Vose:Eli Lilly and Company; Epizyme, Kite, Loxo, Novartis: Research Funding; AbbVie, MEI Pharma: Consultancy. Fakhri:AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genetech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab/Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; LOXO/Lilly: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hoffmann:Kite: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria. Hernandez-Ilizaliturri:Incyte/Morphosys: Consultancy; Collectar: Consultancy; Gilead: Consultancy; BioGene: Consultancy; ADC Therapeutics: Consultancy; Novartis: Consultancy; Epizyme: Consultancy; Dava Oncology: Consultancy; AbbVie: Consultancy; BMS: Consultancy; Amgen: Consultancy; Kite: Consultancy. Zelenetz:None other than mutual funds (401K): Current equity holder in publicly-traded company; AstraZeneca: Consultancy, Honoraria; Abbvie: Research Funding; Pharmacyclics: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Lymphoma Research Foundation: Membership on an entity's Board of Directors or advisory committees; SAB: Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria; MEI Pharma Inc: Consultancy, Honoraria, Research Funding. Kumar:Pharmacyclics: Research Funding; Kite Pharma: Consultancy; Adaptive Biotechnologies: Research Funding; Loxo/Lily Oncology: Consultancy, Research Funding; Beigene: Research Funding; Seattle Genetics: Research Funding; Astra Zeneca: Consultancy, Research Funding; Abbvie Pharmaceuticals: Research Funding; BridgeBio: Current equity holder in publicly-traded company; Janssen: Consultancy; Genentech: Consultancy, Research Funding; Celgene: Research Funding. Munir:AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Tsai:Eli Lilly and Company: Current equity holder in publicly-traded company; Loxo@Lilly: Current Employment. Balbas:Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Liu:Eli Lilly and Company: Current Employment. Ruppert:Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company; Telios Pharma: Consultancy. Nguyen:Loxo@Lilly: Current Employment. Roeker:PeerView: Other: CME speaker; Curio: Other: CME speaker; Ascentage: Consultancy; AbbVie: Consultancy, Research Funding; Adaptive Biotechnologies: Research Funding; Beigene: Consultancy; AstraZeneca: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; DAVA: Other: CME speaker; Janssen: Consultancy; Loxo Oncology: Consultancy, Other: travel support, Research Funding; Medscape: Other: CME speaker; Pharmacyclics: Consultancy; TG Therapeutics: Consultancy; Abbott Laboratories: Current equity holder in publicly-traded company; Genentech: Research Funding; Aptose Biosciences: Research Funding; Dren Bio: Research Funding; Qilu Puget Sound Biotherapeutics: Research Funding. Wang:Bristol Myers Squibb: Consultancy, Honoraria; BioInvent: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Imedex: Honoraria; Hebei Cancer Prevention Federation: Honoraria; Epizyme: Consultancy, Honoraria; Clinical Care Options: Honoraria; BGICS: Honoraria; Anticancer Association: Honoraria; Vincerx: Research Funding; Molecular Templates: Research Funding; Loxo Oncology: Consultancy, Research Funding; Juno Therapeutics: Research Funding; Genentech: Consultancy, Research Funding; Celgene: Other: Travel, Research Funding; WebMD: Honoraria; Studio ER Congressi: Honoraria; Scripps: Honoraria; Practice Point Communications (PPC): Honoraria; Physicians Education Resources (PER): Honoraria, Other: Travel; OncLive: Honoraria; Oncology Specialty Group: Honoraria; Nurix: Honoraria; NIH: Honoraria; Moffit Cancer Center: Honoraria; MJH Life Sciences: Honoraria; MD Education: Honoraria; Meeting Minds Experts: Honoraria; Medscape: Honoraria; IDEOlogy Health: Honoraria; i3Health: Honoraria; Genmab: Honoraria, Research Funding; Eastern Virginia Medical School: Honoraria; Dava Oncology: Honoraria, Other: Travel; CAHON: Honoraria; Bantam Pharmaceutical: Honoraria; VelosBio: Consultancy, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Pepromene Bio: Consultancy; Parexel: Consultancy; Oncternal: Consultancy, Research Funding; Milken Institute: Consultancy; Miltenyi Biomedicine: Consultancy; Merck: Consultancy, Honoraria; Eli Lilly and Company: Consultancy, Research Funding; Leukemia & Lymphoma Society: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; InnoCare: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; DTRM Biopharma (Cayman) Limited: Consultancy; Deciphera: Consultancy; Be Biopharma: Consultancy; AstraZeneca: Consultancy, Honoraria, Other: Travel, Research Funding; Amphista Therapeutics Limited: Consultancy; ADC Therapeutics America: Consultancy; CSTone: Consultancy; Practice Point Communications: Honoraria; Physicians Education Resources: Honoraria; Acerta Pharma: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; TS Oncology: Honoraria; Mumbai Hematology Group: Honoraria; OMI: Honoraria; Pharmacyclics: Honoraria.
Pirtobrutinib is approved for MCL treatment by FDA in USA to treat relapsed or refractory MCL after at least two lines of systemic therapy including prior BTK inhibitor treatment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal